Model I: Chassis
Notations & Assumptions
The notations of the parameters and variables are given as follows:
| Parameters | ||||||||
|
||||||||
| Variables | ||||||||
|
Overall Explanations of the Model
According to microbic growth process:
- Algae Growth Dynamics
- Growth and Stationary
- Dying(Apoptosis)
- Biological stability analysis
- AGER(Product) Generation Dynamics
- Chromoprotein Production and Disease Reporting
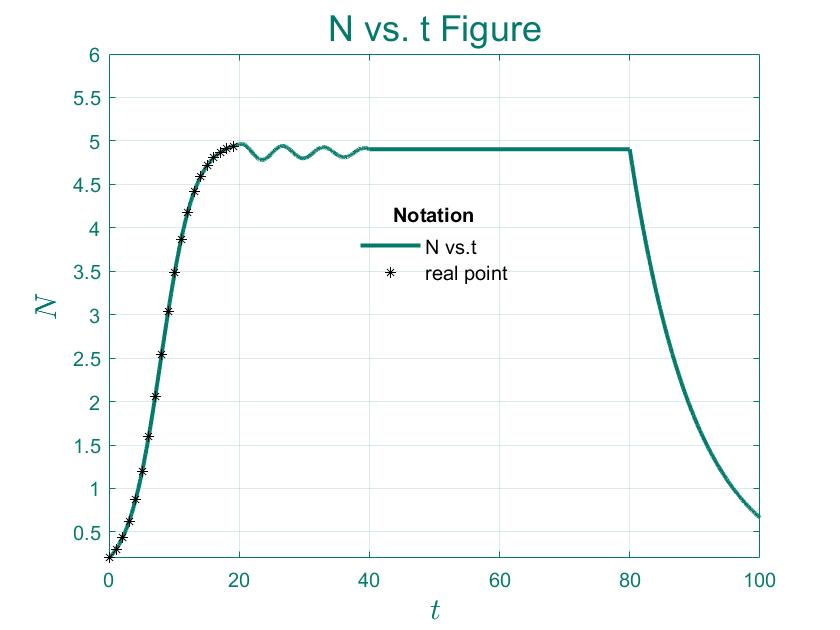
Algae Growth Dynamics
Since space and resources are limited, it is impossible to support an infinite number of algaes. When the population is too large, the birth rate decreases and the mortality rate increases due to the decrease in the average resource occupancy of algaes and the deterioration of the environment.
$N$ denotes the current population size. Let the limitation of the population size that the environment sustain be $N_{max}$. When $N < N_{max}$, the environment can still sustain the growth of population size, and as the population size keeps increasing and approaches $N_{max}$, the inhibitory effect will become more and more obvious. Then, according to the above, $N_{max}-N$ can exactly represent the number of populations that the environment can sustain,$r$ represents the possible max relative growth speed of the algaes, and $t$ represents time.
Considering the solution to this OED:
Solving this ODE:
Definition (Autonomous System)
The differential equation in the following form:
The differential equation in the following form: $\frac{dx}{dt} = f(x)$ is called autonomous systems, where $f(x)$ does not obviously contain a time variable. Clearly, ‘Logistic Model’ is an autonomous system.
Definition (Phase Plane)
The phase space of the autonomous system $\frac{dx}{dt} = f(x)$ is the space $\mathbb{R}^n$ with ($x_1, · · · , x_n$) as coordinates. In particular, when $n = 2$, the phase space is called the phase plane. The set of points $ \{(x_1, · · · , x_n)|x_i = x_i(t)\}$ in the space $\mathbb{R}^n$ satisfies $\frac{dx}{dt} = f(x)$, (where $i = 1,··· ,n$) called the track line of the system. The distribution of all track lines in phase space is called phase diagram.
Definition (Stability Determination:Lyapunov)
Let $x_{0}$ be the equilibrium point of $\frac{dx}{dt} = f(x)$:
- $x_{0}$ is stable for any given $\varepsilon>0$,there exists a $\delta>0$ such that whenever $|x(0)-x_{0}|<\delta$, there is $|x(t)-x_{0}|<\varepsilon$,which holds for all $t$.
- $x_{0}$ is asymptotically stable if it is stable and $\mathop{lim}\limits_{t\rightarrow\infty}|x(t)-x_{0}|=0$.
- $x_{0}$ is unstable if the first condition above is not satisfied.
Definition
Let $x^{0}$ be the equilibrium point of the differential equation $\frac{dx}{dt} = f(x)$. If $f'(x^{0}) < 0$, then $x^{0}$ is asymptotically stable; if $f'(x^{0})>0$, then $x^{0}$ is unstable.
Proof
According to Taylor Expansion:
- Because $x^{0}$ is the equilibrium point, so it is obvious that $f(x^{0})=0$.
- If $f'(x^{0}) < 0$, when $x < x^{0}$, then $f(x)>0$, $x$ monotonic increase; when $x>x^{0}$, then $f(x) < 0$, $x$ monotonous reduction, all above indicates that $x\rightarrow x^{0}$.
- If $f'(x^{0})>0$, similar analysis process.
For Logistic Model:
It's obvious when $N=N_{max}$, it is asymptotically stable.
AGER(Product) Generation Dynamics
Considering algaes growth and product generation as a mixed model, that is, related to both primary and secondary products(related to both cell accumulation and its growth rates).
Chromoprotein Production & Disease Reporting
Considering the rate of change of activated cells depends on the cell growth rate, the activated cell number and the amount of cell accumulation.
Under the assumption that the production of chromoprotein is proportional to the number of activated cells, the amount of chromoprotein production can be reflected by the number of activated cells.
Model II: Hydrogel & Drug
Hydrogel Phase Transition & Swelling
Hydrogel Drug Release
The model considers the phenomenon of drug diffusion as well as polymer relaxation, assuming that the drug release is a fast diffusion-controlled phenomenon and subsequently controlled by polymer chain relaxation, and the whole drug release process considers the superposition effect of two components.
It is preferred to use Scaling Law to determine whether this Model is suitable.
It is admitted that much of the data of the detailed model is very difficult to measure.
the Whole Process of Hydrogel Swelling & Drug Release
Kinetic processes are dynamic evolutionary processes that characterize the movement of matter from one equilibrium state to another.
Model III: Drug Absorption
Notations & Assumptions
The notations of the parameters and variables are given as follows:
| Parameters | ||||||||
|
||||||||
| Variables | ||||||||
|
Pharmacodynamics
The way in which drugs in biological dressings are absorbed into the body is often different from the rapid intravenous injection and constant rate intravenous drip processes.
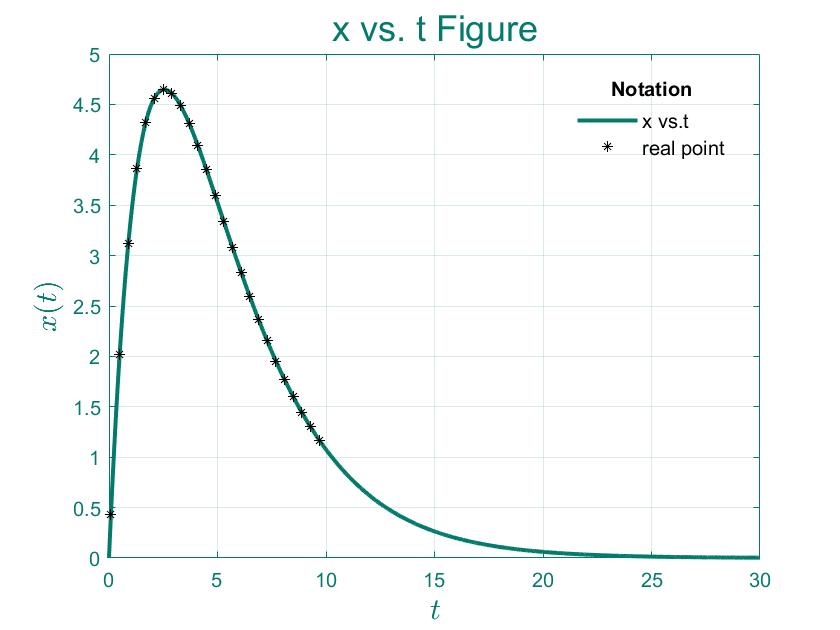
After application, the drug is generally concentrated on a certain part of the body and is gradually absorbed by the contact between its surface and the muscle. Let the rate at which the drug is absorbed be proportional to the amount of the stock drug, and note the proportionality factor as $k_{1}$. That is, if the amount of drug remaining at moment $t$ is noted as $y(t)$, then $y$ satisfies:
where $y(0) = R$, represents the total amount of drug entering the body.
Single-compartment model of drug distribution: Assume that the drug in the body is uniformly distributed at any moment, and let the total amount of drug in moment $t$ be $x(t)$; the system is in a dynamic equilibrium, the following relationship is assumed:
The rate of drug breakdown and excretion is usually considered to be proportional to the current concentration of the drug (note the proportionality factor as $k_{2}$), which is usually considered $(\frac{dx}{dt})_{out}= k_{2}x$, but the input pattern of the drug depends on the mode of administration. Considering the nature of drug delivery by application:
Solve the above ordinary differential equation: \begin{equation} x(t) = \frac{k_{1}R}{k_{1}-k_{2}}(e^{-k_{2}t}-e^{-k_{1}t}) \end{equation}
So the drug concentration in the blood is $C(t) = \frac{x(t)}{V}$, $V$ is the volume of blood.
Definition (Analysis)
Normally,there is always $k_{1}>k_{2}$ because the rate of input is generally greater than the rate of breakdown and excretion before the drug is absorbed, but there will be exceptions. At $k_{1}>k_{2}$ the amount of drug in the body is not very large, which in medical terms is called flip-flop; when $k_{1}=k_{2}$ , the solution of the equation can be considered as an undefined equation of type$\frac{0}{0}$, then L'Hospital's rule can be used to obtain when $k_{2}\rightarrow k_{1}$, the blood drug concentration is $C(t) = \frac{k_{1}R}{V}te^{-k_{1}t}$
REFERENCES
- [1] Cheng, R., Feng, F., Meng, F., Deng, C., Feijen, J., Zhong, Z., 2011. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. Journal of controlled release 152, 2–12.
- [2] Chiu, L.L., Radisic, M., 2011. Controlled release of thymosin β4 using collagen–chitosan composite hydrogels promotes epicardial cell migration and angiogenesis. Journal of Controlled Release 155, 376–385.
- [3] Killion, J.A., Geever, L.M., Devine, D.M., Kennedy, J.E., Higginbotham, C.L., 2011. Mechanical properties and thermal behaviour of pegdma hydrogels for potential bone regeneration application. Journal of the mechanical behavior of biomedical materials 4, 1219–1227.
- [4] Parimala, K., Sudha, M., 2012. Effect of hydrocolloids on the rheological, microscopic, mass transfer characteristics during frying and quality characteristics of puri. Food Hydrocolloids 27, 191–200.

